
 |
|
EudraVigilance Data Analysis System The EudraVigilance Data Analysis System has been designed to allow users to analyse safety data collected in EudraVigilance in view of allowing better-informed decisions about the safety profile of medicinal products. It provides pharmacovigilance and Clinical Trials departments with a range of analytical tools: from measuring reporting compliance for regulatory purposes, to pharmacovigilance analyses (such as signal detection tools). The following illustration displays the components of the EudraVigilance
Data Analysis System: 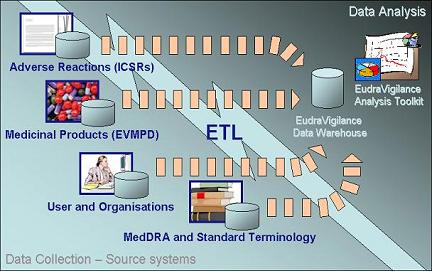
This architecture has the following components:
Source Systems Source systems capture and store the data that are reported to or
used by EudraVigilance. These systems include the EudraVigilance
Database Management system (EVDBMS), the EudraVigilance Medicinal
Product Dictionary (EVMPD), the EudraVigilance registration database,
the Medical Dictionary for Regulatory Affairs (MedDRA) and other
internationally agreed standard terminologies used within EudraVigilance
(e.g. European Pharmacopoeia Dosage Forms).
The EVMPD contains information on:
The EudraVigilance registration database contains information about National Competent Authorities, Marketing Authorisation Holders, and Sponsors of clinical trials in the EEA that have electronic reporting obligations and are registered with the EudraVigilance system. This information is used to configure data access rights and privileges in the EudraVigilance Data Analysis System. ETL Process The extraction, transformation, and loading (ETL) process is the means by which data is transferred from source systems and loaded into the EudraVigilance Data Warehouse. Specifically, the ETL process does the following:
The ETL process is done nightly so that every day the EudraVigilance Data Warehouse is populated with updated data from the day before. EudraVigilance Data Warehouse The EudraVigilance Data Warehouse is the repository for storing the information required to analyse data from the disparate
source systems involved in the EudraVigilance Data Analysis System.
Just as the source systems are designed to process capturing and
storing data, a Data Warehouse is optimised to allow users to
report on and manipulate data. Manipulation of data includes transformation
of variables, filtering and tabulation. EudraVigilance Analysis Toolkit The EudraVigilance Analysis Toolkit provides many predefined table and graph formats for report presentation. Users can customise formatting to suit particular needs, and have a variety of functions available for the manipulation of report results. For example, users can sort and filter data, add subtotals, create new calculations, change the arrangement of data on a report, and more. The way users interact with the EudraVigilance Data Analysis Toolkit is through a graphical user interface (GUI), which will be available via a common web browser. The toolkit GUI can change layout, depending on specific Internet browser (i.e. Netscape or Internet Explorer). A 2 day training course on how to use EVDAS is held 5 times a year at the EMA for members of National Competent Authorities (NCAs). For upcoming training dates please see the Training section. Experts are kindly requested to register at least 3 weeks before the course starts For any further information, please visit the EMA Service Desk portal
|
|
 |
 |
 |